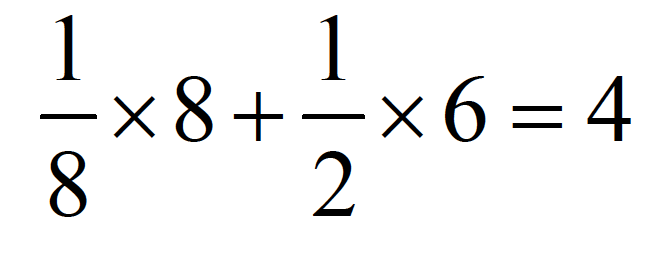Share and Cite
Chicago/Turabian Style
Yun-Hua Liou, and Chih-Chun Lai, "Study on the Influence of Copper Oxide on the Saturation of Porcelain Glaze: A Case Study of Green Cyan Glaze." JDSSI 3, no.1 (2025): 1-12.
AMA Style
Liou YH, Lai CC. Study on the Influence of Copper Oxide on the Saturation of Porcelain Glaze: A Case Study of Green Cyan Glaze. JDSSI. 2025; 3(1): 1-12.
© 2025 by the authors. Published by Michelangelo-scholar Publishing Ltd.
This article is published under the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International (CC BY-NC-ND, version 4.0) license (https://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited and not modified in any way.
3. Results and Discussion
In this experiment, the mineral polymerization ratios of light green and medium green cyan glazes were initially determined while analyzing the conditional factors that influence the texture of glass porcelain.
3.1. The Porcelain Effect of Hill Green Glaze
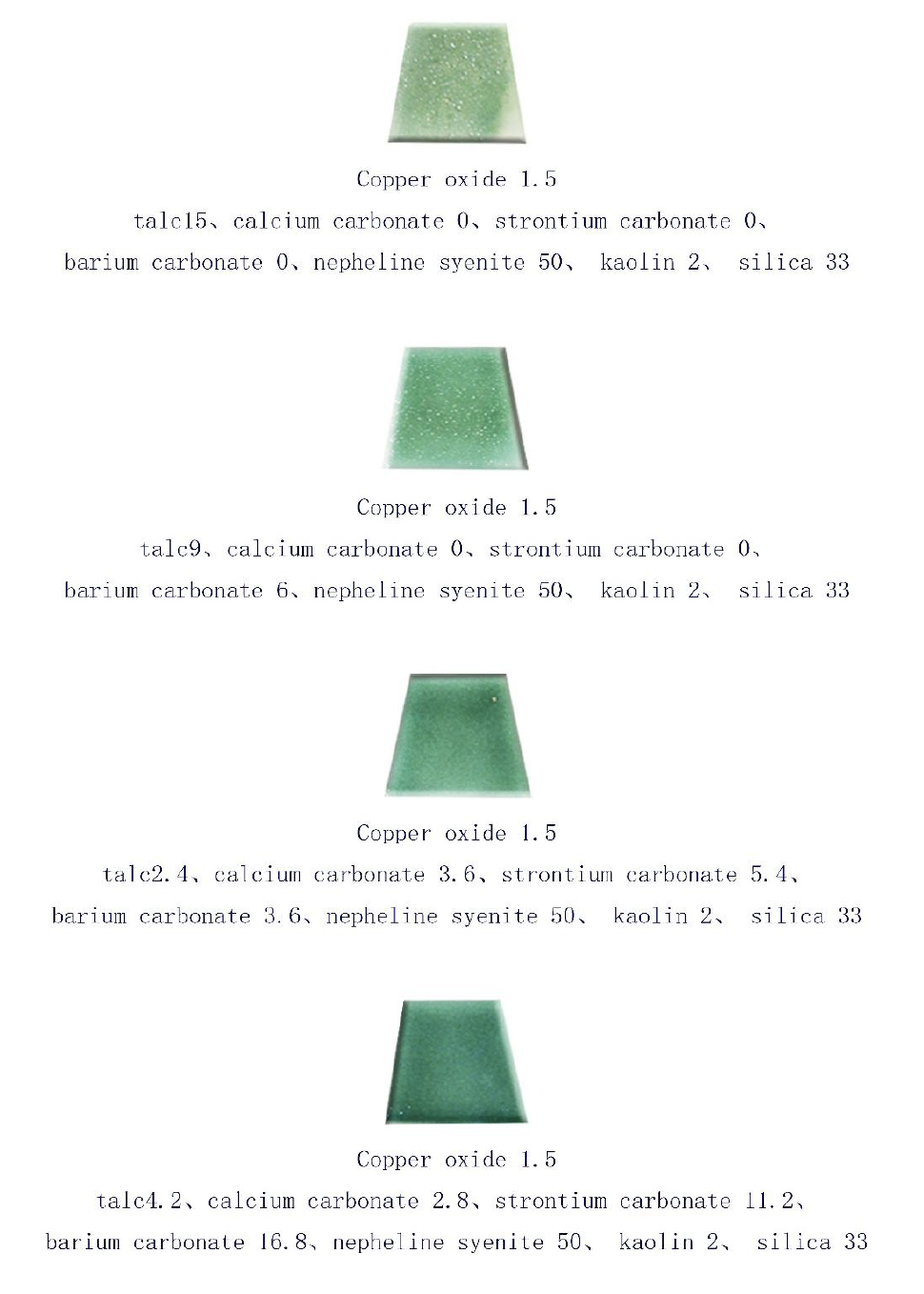
This series of ceramic test pieces transitions gradually from bean sprout green and plain green to the rich glaze color of hill green, encompassing various shades of green. The initial stage of the research revealed that when only talc is used as a flux, without any additional fluxes, the porcelain effect appears rough. At this temperature (1220°C), the glaze is thick and immature, resembling stoneware. It is in a devitrified state, densely covered with pinholes, and exhibits poor color quality (Figure 6).
Study on the Influence of Copper Oxide on the Saturation of Porcelain Glaze: A Case Study of Green Cyan Glaze
1.2. Research Scope
The careful selection of popular colors is essential for enhancing product aesthetics. Beyond merely setting trends, it can significantly boost sales. For instance, in products such as personal accessories (like watches), communication devices (such as mobile phones), and everyday items, the incorporation of unique mineral elements can reveal innovative features. Consequently, many individuals have developed an interest in exploring the field of glaze research.
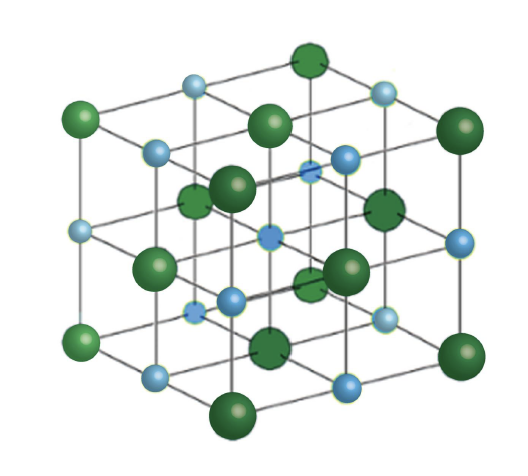
Each of the eight vertices of the crystal contains one-eighth of a chloride ion (Figure 2), while each of the six faces contains approximately one-half of a chloride ion [3]. Therefore, the formula for calculation is:
Table of Contents
- Abstract
- Introduction
- Experimental Procedures and Literature Review
- Results and Discussion
- Conclusions
- Author Contributions
- Funding
- Acknowledgements
- Conflicts of Interest
- Author Biographies
- References
1. Introduction
During the development of Eastern ceramic crafts across various dynasties, artisans inadvertently discovered the properties of different alkaline earth metals, which led to the transformation of glazes after high-temperature sintering. Researchers have identified a diopside glass system that enhances the sintering capabilities of glass-ceramics, resulting in luminous and captivating glazes.
1.1. Research Purpose
This study aims to determine the optimal copper glaze ratio for the green-cyan color system. By narrowing the firing temperature range, researchers can identify the ideal glaze color from various sets of experimental test pieces. This information serves as a valuable reference for creators interested in exploring this color system and experimenting with blends of mineral elements. The glaze firing process must elevate the ignition point within the kiln [1]. By consuming substantial amounts of oxygen, time, and energy, the minerals can be purified and crystallized to achieve the desired glaze color.
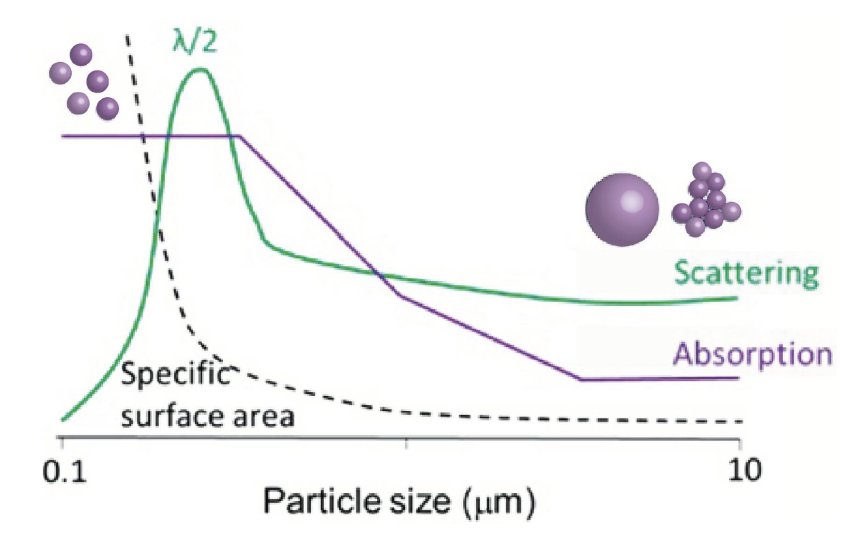
If the specific surface area of the crystal is small, the glaze will appear brighter because the reflective area for the light source is larger. This configuration enhances the saturation of the glaze color [2]. The agglomerated particles can be broken down into smaller sizes, resulting in improved tint strength, as previously discussed. Additionally, the irregularities in particle shape contribute to the lower reflectance observed in samples with low tint strength (Figure 1). Both ceramic and glass art have a long and rich history that spans thousands of years, reflecting the flourishing of art and culture. This enduring legacy is attributed to the remarkable versatility of these materials and their manufacturing processes, which enable the creation of vibrant glaze colors.
3.3. Summary of the Effects of Glaze Color Concentration
In experiments involving alkaline earth metal oxide formulations containing only strontium carbonate and barium carbonate, the concentrations of copper oxide were adjusted to 1.5%, 2.5%, and 3.5%, respectively. During the firing process, it was observed that the glaze color of the specimens transitioned from light to dark [15]. Mr. Allesch, a German artist, argued that there are variations in human preferences for monochromatic and multicolor schemes regarding single-color and multi-color matching. These variations exist not only among individuals but also in the relationships between monochromatic and multicolor pairings.
Color relationships characterized by high luminance and high purity produce strong stimulation for the human senses and are more likely to be favored by individuals [16]. Ceramic craftsmanship is increasingly being recognized as a significant component of the art world, where it is appreciated not merely as a craft but as a legitimate form of artistic expression [17]. In the creation of modern ceramic art, decorative ceramics that utilize texture as the primary decorative technique play a crucial role. The diverse methods of texture creation, combined with a variety of glaze colors, result in an abundance of texture types that are both rich and adaptable.
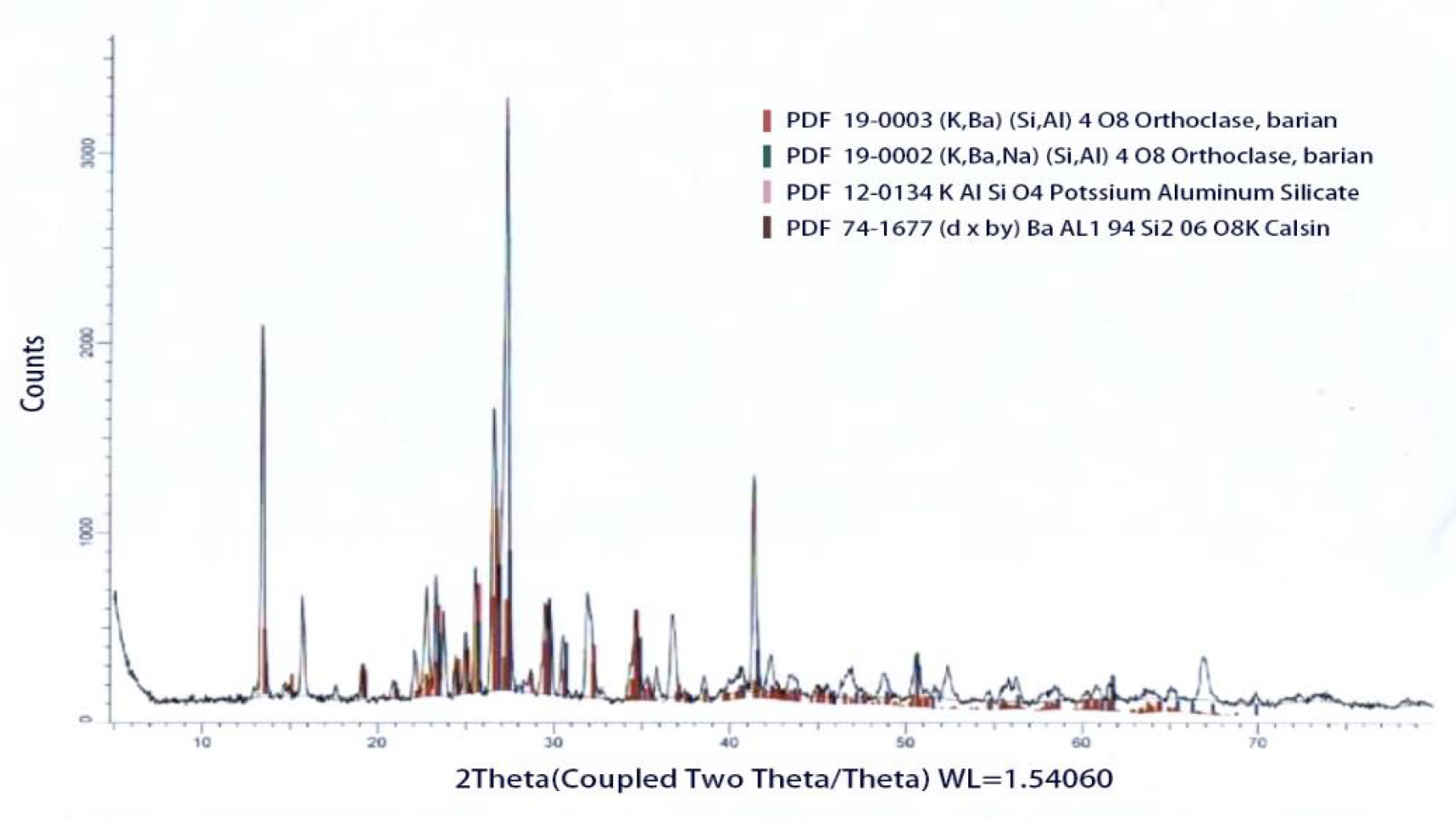
The conclusion is that when the alkaline earth metal oxide content reaches 15%, the glaze color transitions to a hilly green, initially characterized by a copper oxide concentration of 1.5%. As the copper oxide concentration increases to 2.5% and 3.5%, the glaze color gradually deepens and darkens, resulting in shades of deep green and forest green. Therefore, the concentration of copper oxide significantly influences the depth of the glaze color. Evidence of the distribution of glaze components can be obtained from the X-ray diffraction (XRD) crystal image [18]. X-ray diffraction analysis reveals the long-range order (i.e., the structure) of crystalline materials and the short-range order of non-crystalline materials.
4. Conclusion
The sintering temperature of the test piece in this experiment was set within the medium-high temperature range of 1200°C to 1220°C, where it was oxidized and sintered with copper glaze (less than 3.5%) [19]. The colors produced are influenced by the burning conditions. This research analyzes the characteristics of ionic crystals from a scientifically designed perspective and employs the mechanism of copper oxide formation to provide a systematic model that can serve as a reference for other studies on mineral ratios [20]. The formative discourse can derive colors from existing hues, as each artistic effect necessitates a visible and tangible form. Consequently, the formative discourse relies on sensory engagement and human experience. The key points of this glaze science experiment are summarized below:
- Lowering the firing temperature of the glaze can reduce oxygen consumption and promote a more environmentally friendly process.
- By utilizing copper oxide as the primary colorant in the glaze and adjusting the proportions of alkaline earth metals in the composition, a novel blend of ionic crystals has been developed.
From the laser crystal image, we can clearly observe the proportional distribution of glaze components after high-temperature firing (Figure 8). The polymerization of these glaze components occurs at different firing temperatures and through various molding methods, prompting many contemporary potters to incorporate these diverse mineral elements into their ceramic works. This approach not only provides a wider range of options but also facilitates collaboration among colleagues interested in developing glazes, as well as individuals with expertise in various fields. The ultimate goal is to promote a more environmentally friendly porcelain process that reduces the required firing time while producing more vibrant and saturated glaze colors.
Talc and calcium carbonate exhibit a greenish tint, while strontium carbonate and barium carbonate display a bluish-green tint.
2.1. Temperature Control in Porcelain Sintering
In this experiment, oxidation sintering was employed [12]. It is well established that the color produced by incorporating a small amount of iron oxide (Fe2O3) into a base glaze fired in a reduction atmosphere is referred to as celadon. The resulting glaze can exhibit a range of colors, including yellow, tan, gray, gray-green, light green, olive green, and blue-green (jade green). The specific hue of celadon is influenced by the concentration of iron oxide present, as well as the ratios of Fe2+ and Fe3+, which directly affect the glaze color depending on the firing conditions.
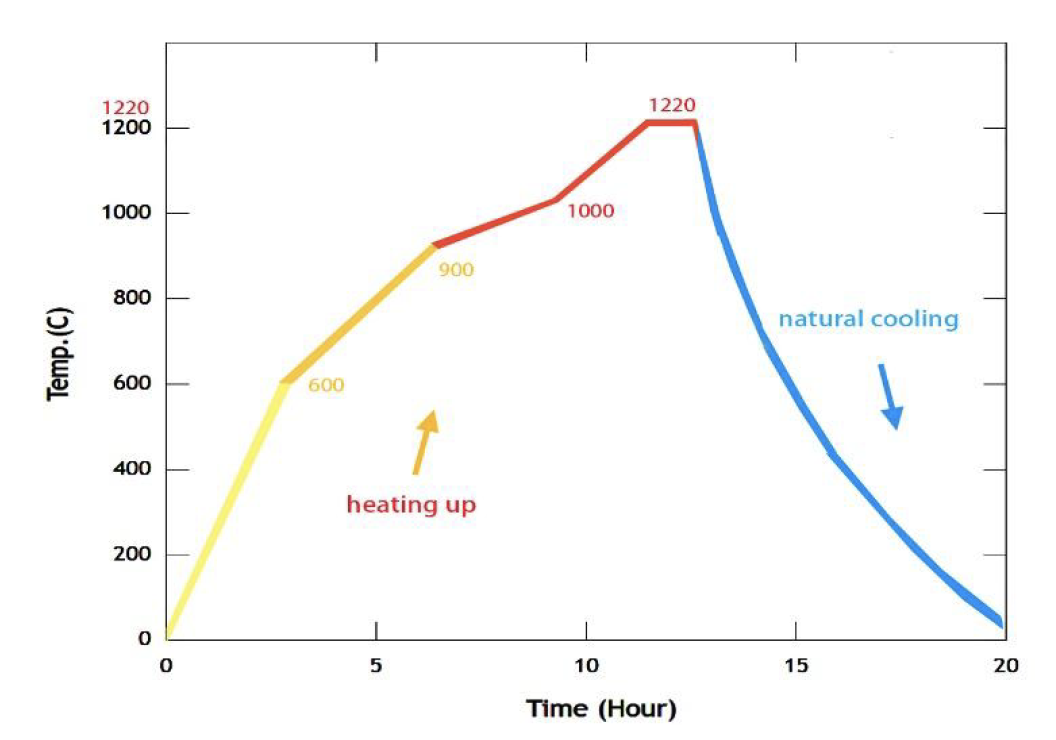
As a sintering testing machine, the electronic kiln follows a specific temperature control curve. The first stage involves heating from 0 to 600 degrees Celsius over a duration of 3 hours. The second stage raises the temperature from 600 to 900 degrees Celsius for 2 hours. The third stage increases the temperature from 900 to 1000 degrees Celsius for 3 hours. The fourth stage involves heating from 1000 to 1220 degrees Celsius for 2 hours, and the fifth stage maintains a temperature of 1220 degrees Celsius for 1 hour (Figure 5). This sintering process, along with the specific gravity of the heating or cooling minerals in the formula, typically requires adjustments to the glaze composition based on the temperature control operator’s personal experience, similar to that of a traditional craftsman. The reactions involved in the glaze coloring process originate from the cations of the metal-organic complexes present in the formula. Stoneware is a broad term that refers to clays with a melting or maturity point ranging from 1,100°C to 1,200°C. In addition to its high firing temperature [13], stoneware is often blended with other minerals, which create denser pores compared to traditional clay, resulting in rock-like strength. This type of stoneware is commonly referred to as stoneware and is widely utilized in both industrial settings and independent studios due to its relatively low cost and durability. Furthermore, stoneware is highly versatile in terms of pricing, applications, and processing methods. The ideal porcelain glaze can only be achieved after the matrix is diffused into the glass body and subjected to high-temperature heat treatment.
2.2. Research and Development Analysis of Porcelain Glaze Colors
Through the fusion and transformation of high-temperature ionic crystals, a vibrant culture is cultivated by artists and companies. Iron glaze, a selective chromophore that varies based on the amount of iron and the firing conditions, is essential for the creation of traditional ceramic art. Ceramic artist Mr. Sun Chao’s book, BORN BY FIRE [14], Glaze and kiln firing as some of the oldest artistic materials, which are also among the most variable and unpredictable. Throughout history, China has developed a diverse range of representative glaze colors. Consequently, the ingredients and unique glaze formulas of certain ceramic brands are considered confidential and proprietary technologies that must remain undisclosed.
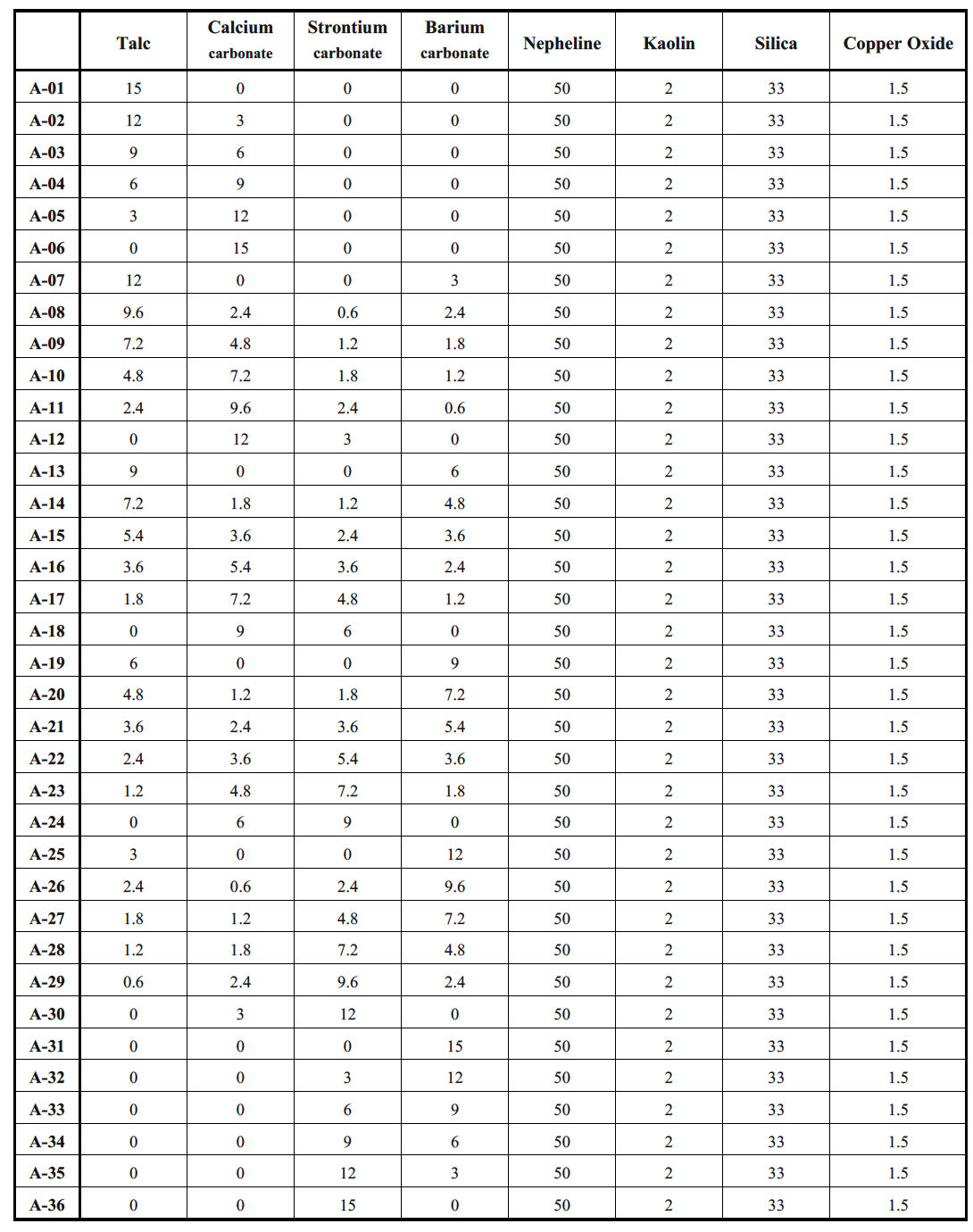
In this experiment, 50 grams of nepheline feldspar, 2 grams of kaolin, and 33 grams of silica are utilized. Each sample exhibits varying ratios of mineral increases and decreases. The initial experimental formula incorporates a 1.5% proportion of copper oxide [11]. The different ratio values are primarily documented through a recording method, in which the ceramist notes the varying ratios. After the porcelain blanks have naturally dried, the physical properties, stress characteristics, and styles of the finished products are meticulously recorded for use as reference models.
After completing the initial stage of the firing experiment, various shades of glaze colors were identified, and optimal basic conditions that enhance gloss were selected. The next step involves conducting tests on color saturation and concentration using copper oxide concentrations of 1.5%, 2.5%, and 3.5% for group analysis (Figure 4).
3.2. The Porcelain Effect of Forest Green Glaze
The concentration of copper in this group of ceramic test pieces increases to 2.5% and 3.5%. Consequently, the green saturation of the glaze intensifies, resulting in a deep forest green color (Figure 7).
Abstract: The science and aesthetics of glaze color represent a unique cultural art form. This study investigates how adjusting the ratio of cyan glaze components and controlling the lattice sintering temperature at approximately 1210 degrees Celsius can produce variations in shades of hill green and forest green cyan glazes on white porcelain test pieces. In Chinese, the term originally refers to green, but it is often used to describe the appearance of mountain scenery from a distance, particularly in gray and rainy weather. In such conditions, the green hues blend into blue, subtly revealing the beautiful scenery of distant mountains. The experimental results regarding glaze lattice formation indicate that test pieces containing talc and calcium carbonate exhibit a greenish tint, while those made with strontium carbonate and barium carbonate display a bluish-green tint.
Through the innovative combination of copper oxide minerals and precise control of firing temperature within the medium to high-temperature range, the fusion and transformation of ionic crystals can be achieved, while simultaneously reducing oxygen and energy consumption in the process. This study introduces a novel method of blending minerals to investigate the mechanism behind altering the glaze color recognition threshold. These findings hold academic significance for understanding the impact of alkaline earth metals on green-cyan glaze colors and also present new avenues for future research in the field of glaze coloration.
by
Yun-Hua Liou 1, 2*,
1,Chih-Chun Lai 1
1. Institute of Design Science, Tatung University, Taiwan
2. Department of Creative Product Design, Hungkuo Delin University of Technology, Taiwan
* Author to whom correspondence should be addressed.
JDSSI. 2025, 3(1), 1-12; https://doi.org/10.59528/ms.jdssi2025.0109a28
Received: August 13, 2024 | Accepted: November 5, 2024 | Published: January 9, 2025
In the ceramic arts, it has been discovered that intense heat can transform clay into a highly durable material [4]. The invention of glazes makes vessels impervious to liquids. Clay, when fired at a wide range of temperatures—from earthenware to porcelain—can be enhanced through appropriate glazing and firing techniques. From this point onward, the exploration of the practical applications of this material became closely intertwined with the development of its aesthetic potential. The use of high-stability, unique brand-specific colors is expected to remain a key focus in the research and development efforts of artists, the ceramic industry, and companies that prioritize corporate branding.
The white porcelain test pieces were coded, resulting in a total of 36 pieces. The same glaze composition was then soaked separately, using different glaze proportions. A total of 36 pieces were designated from A-01 to A-36, with the first group of glaze test pieces referred to as Group A.
Author Contributions
Yun-Hun Liou: Writing—original draft; Chih-Chun Lai: Writing—review and editing.
Funding
Not applicable.
Acknowledgements
The authors would like to express their gratitude to Director Xu Yan from Datong University, Teacher Li Songwan from the New Taipei City Glaze Association, and Dean Lin Youyi from Hungkuo Delin University of Technology for their ongoing support.
Conflicts of Interest
The author declares that they have no conflicts of interest related to this research.
Author Biographies
Yun-hua Liou earned a master’s degree from the Industrial Product Design Institute at Shih Chien University and currently serves as an assistant professor at Hongguo Delin University of Science and Technology. With over a decade of industrial experience, he has contributed to product R&D and electronic painting in companies such as Haitai Technology and Pusheng Digital Technology. Transitioning to academia in 2013, he has focused on teaching design and electronic painting. In 2023, he joined the doctoral program in design science at Datong University, aiming to integrate craft creation with digital technology.
Dr. Chih-Chun Lai received the Ph.D. degree in Design Science from Tatung University, Taipei, Taiwan.He has been a Professor with the Industrial Design Department, Tatung University. From 2002 to 2006, he was a Patent Agent with Taiwan Intellectual Property Office. His research interests include product design, ergonomics, multimedia, color, social culture and patent research. Since 2012, he has been the advisors for the students winning Golden Pin Design Awards of Young Designers’ Exhibition (YODEX) in Taiwan, and many international design awards, including a Red Dot: Best of the Best and iF Design Talent Awards. He is a Project Investigator with the National Science and Technology Council (NSTC, Taiwan).
2. Experimental Procedures and Literature Review
In the East, the development of glaze formulas and product colors that reflect unique brand characteristics necessitates the incorporation of various oxidized metals as color developers, in addition to mineral elements. These metals include chromium, chromium oxide, iron, iron oxide, cobalt, cobalt oxide, copper, and copper carbonate, among others [5]. When using iron oxide and loess as raw materials for iron sources, the resulting colors are sky green and olive green for the loess sample, and a hue resembling bean green for the slag sample. The glaze of iron oxide samples contains numerous quartz crystals, which contribute to high turbidity. The size of the liquid-liquid phase separation is larger near the quartz crystals, while it diminishes with increasing distance from them. Additionally, it is essential to raise the ignition point in the kiln. After exposure to high temperatures, significant oxygen consumption, and prolonged energy use, the glaze must undergo a purification and crystallization process to achieve the desired color.
These heating processes require temperatures exceeding 1,300 degrees Celsius, with some elements necessitating temperatures as high as 1,600 degrees Celsius to induce changes in the ionic crystals that create the distinctive luster effect of porcelain glass [6]. The protective coating deposited through ion-plasma sputtering exhibits vivid aesthetic and decorative properties, making it an attractive solution for design challenges. Additionally, it enhances the operational characteristics of the products by increasing their strength, hardness, and durability [7]. Japan Seiko Ink has developed a range of innovative annual product color systems that utilize various mineral elements. These systems will be employed until the release of the iPhone 16 series in 2024.
These mineral elements require elevated sintering temperatures to enhance the degree of crystallization in the glaze. For example, the yellow glaze is derived from titanium (Ti), the blue glaze is obtained from cobalt (Co), the green glaze is produced from zinc oxide (ZnO), and the dark green glaze is sourced from zinc trioxide. The black glaze, in conjunction with dichromium (Cr2O3), is refined and fired using copper, chromium, iron, and other minerals [8]. Comparatively small amounts of titanium (Ti), manganese (Mn), copper (Cu), and even cobalt (Co) can significantly influence color. The mineral polymerization described above must undergo a high-temperature process to achieve the desired glaze color and saturation. This research introduces a novel scientific method for studying the morphological changes of various mineral elements following porcelainization within the medium to high-temperature range. This is accomplished by adjusting the glaze formula and controlling the sintering temperature to approximately 1200 degrees Celsius. The experimental results from this glaze design not only document and preserve the craft culture of modern glaze science in Taiwan but also significantly reduce the glaze firing time and simplify the complex experimental process (Figure 3).
Each atom has six nearest neighbors, with octahedral geometry. This arrangement is known as cubic close packed (ccp) or face-centered cubic (fcc). Light blue = Na+ Dark green = Cl -.
Research papers on a value strategy by combining traditional craft and modern technology emphasize the creation of unique and stable ceramic glaze colors [9]. The glaze colors of Chinese pottery have evolved from the Qin and Han Dynasties to the Western Han Dynasty, primarily showcasing brown and yellow hues with a rough texture. By the Eastern Han Dynasty, as well as during the Spring and Autumn Period and the Warring States Period, it was determined that the glaze formula must include oxidized metals, with increased amounts of alumina and iron oxide, along with higher firing temperatures to enhance both the hardness and color saturation of the glaze. Oxidation firing must be conducted at high temperatures of 1300 degrees Celsius, resulting in glaze colors that typically exhibit earthy and natural tones [10]. In modern ceramic art production, coloration is controlled by adjusting the glaze composition, which includes feldspar, lime, silica, and clay, among other materials, as well as the burning conditions (redox conditions during heating and cooling). These factors necessitate the application of high sintering temperatures and extended thermal cycles, which not only elevate production costs but may also compromise the structural integrity of the pigments. In this experiment, four alkaline earth metal oxides—talc, barium carbonate, strontium carbonate, and calcium carbonate—were utilized as variable factors (Table 1).
References
1. Enríquez, E., et al. “Advances and challenges of ceramic pigments for inkjet printing.” Ceramics International 48, no. 21 (2022): 31080-31101. [CrossRef]
2. Sharafudeen, Riyas. “A spectroscopic method for quick evaluation of tint strength and tint tone of titania (rutile) pigment and factors affecting them.” Color Research & Application 44, no. 1 (2019): 44-49. [CrossRef]
3. “Crystal calculation formula.” Wikipedia. https://en.wikipedia.org/wiki/Ionic_crystal.
4. Juntradee, Weeraya. “Possibilities of combining ceramics and recycled glass for decorative lighting design.” Veridian E-Journal, Silpakorn University (Humanities, Social Sciences and arts) 12, no. 1 (2019): 1071-1088.
5. Liu, Pengju, et al. “Analysis of the influence of iron source and its occurrence state on the color of celadon glaze.” Ceramics International 48, no. 13 (2022): 18425-18432. [CrossRef]
6. Chumachenko, Natalia, Vladimir Tyurnikov, and Il’nur Khafizov. “Study of the Glaze Layer Surface of Ceramic Tiles Before and After Ion-plasma Deposition of Protective-decorative Coating.” In IOP Conference Series: Materials Science and Engineering, vol. 661, no. 1, p. 012116. IOP Publishing, 2019. [CrossRef]
7. “What is the difference between iPhone 13 Pine Ridge Turquoise and iPhone 11 Night Green?” MrMad. https://mrmad.com.tw/iphone-13-pro-alpine-green-vs-iphone-11-midnight-green-color.
8. KARASU, Bekir, Ülkü Melda ANDAŞ, and Gizem AK. “Celadon Glazes.” El-Cezerî Journal of Science and Engineering 6, no. 2 (2019): 388-413. [CrossRef]
9. Liou, Yun-Hua, and Zhu, Xujian. “A Value Strategy by Combining Traditional Craft and Modern Technology: Survey on Domestic Ceramic Brand.” 2013.
10. Ojima, Hitoshi, et al. “Evaluation of valencies and local coordination structures of Fe in Kasama celadon glazes.” Journal of the Ceramic Society of Japan 130, no. 3 (2022): 290-293. [CrossRef]
11. Hu, Xiaobing, et al. “Research on the Application of Polymer Materials in Contemporary Ceramic Art Creation.” Polymers 14, no. 3 (2022): 552. [CrossRef]
12. Katsuki, Hiroaki, et al. “A relationship between oxidation state of iron and color of Arita celadon glaze characterized by 57Fe-Mössbauer spectroscopy.” Journal of the Ceramic Society of Japan 122, no. 1426 (2014): 520-522. [CrossRef]
13. Rabbani, Winsa Rizqi, and Dedi Ismail. “Eksplorasi Limbah Kaca Pada Badan Keramik Stoneware.” Jurnal Desain Indonesia 3, no. 2 (September 2021): 1-9. [CrossRef]
14. Sun, Chao. Born by Fire. Business Partner in Taipei, 2001.
15. Allesch, GJ V. “Die aesthetische Erscheinungsweise der Farben.” Psychologische Forschung 6, no. 1 (1925): 1-91. [CrossRef]
16. DeKeyser, Renee, and Nikki Van Es. “How Is Ceramic Craft Transforming Itself to a Form of Contemporary Art?” Academic Skills, Erasmus University Rotterdam, October 27, 2022.
17. Lin, Zibo, and Man Dou. “Texture and Emotional Expression of Modern Ceramic Art.” In 2022 3rd International Conference on Language, Art and Cultural Exchange (ICLACE 2022), pp. 632-636. Atlantis Press, 2022. [CrossRef]
18. Khan, Hayat, Aditya S. Yerramilli, Adrien D’Oliveira, Terry L. Alford, Daria C. Boffito, and Gregory S. Patience. “Experimental methods in chemical engineering: X‐ray diffraction spectroscopy—XRD.” The Canadian journal of chemical engineering 98, no. 6 (2020): 1255-1266. [CrossRef]
19. Ojima, Hitoshi, et al. “Evaluation of Valencies and Local Coordination Structures of Fe in Kasama Celadon Glazes.” Journal of the Ceramic Society of Japan 130, no. 3 (2022): 290-293. [CrossRef]
20. Rashed, Hassanein, M. R. Al-Kizwini, and Bahaa Al-Saadi. “Seen and Unseen Argument in the Contemporary Ceramic Artworks: Study of Post-Modernist Cultural System.” Utopía y praxis latinoamericana: revista internacional de filosofía iberoamericana y teoría social 1 (2021): 90-101.